Abstract
Study Question: What are pre-transplant factors which predict the risk of development of Cytokine Release Syndrome after Haploidentical Peripheral Blood Transplantation?
Significance: Allogeneic hematopoietic cell transplantation is a cornerstone of therapy for hematologic malignancies and often a patient's only curative intent treatment. Overall outcomes for haploidentical transplantation appear to be excellent, however, this novel approach brings toxicities that are particular to its biological and clinical milieu. We recently described occurrence of severe cytokine release syndrome (CRS) after haplo-HCT. Severe CRS was associated with poor clinical outcomes, including transplant related mortality (TRM), overall survival (OS), and neutrophil engraftment (Abboud et al, BBMT, 10/2016).
However, the factors predicting the occurrence severe CRS after haplo-HCT are not known. The diagnosis of CRS requires a high level of clinical suspicion, and early treatment leads to rapid resolution of symptoms. Identifying at-risk patients prior to transplantation is clinically important.
Patients and Methods: All patients (150 consecutive patients) were included who underwent peripheral blood T-Cell replete haplo-HCT with PTCy from July 2009 through October 2016 at Washington University in St. Louis. Since CRS can resemble sepsis syndrome, and additionally sepsis may be a confounding factor by causing CRS independently, patients with a documented infection or clinical suspicion of sepsis were excluded from the analysis (14 patients). Steroid use may also be a confounding variable, therefore, we excluded patients who received steroids between days -5 and the development of CRS (0 patients). Clinical data was missing for only 3 out of the 150 patients, in all cases due to random errors in institutional EMR migration, therefore they were excluded from analysis and we will not undertake imputation of missing values. CRS was graded per the criteria used in our previous work, based on the standards originally proposed by Lee et al, modified to include altered mental status and new onset cardiomyopathy.
Statistical Analysis: An adaptive lasso with SBC selection with split categorical predictors was used to identify 11 predictors of severe CRS (grade 2-4). Model selection was repeated 100 times using resampled (bootstrapped) subsets of the data, and the parameter estimates averaged to obtain an average representation of predictor strength.
Results: The final selection of predictors included CD34+ cells received <5.0, CD34+ cell received > 5.01, T cell dose, HLA match, CMV status of recipient, presence of a prior transplant, donor was a first degree relative, sex of donor, use of myeloablative conditioning, HCT-CI score, T cell dose and age of recipient at transplant.
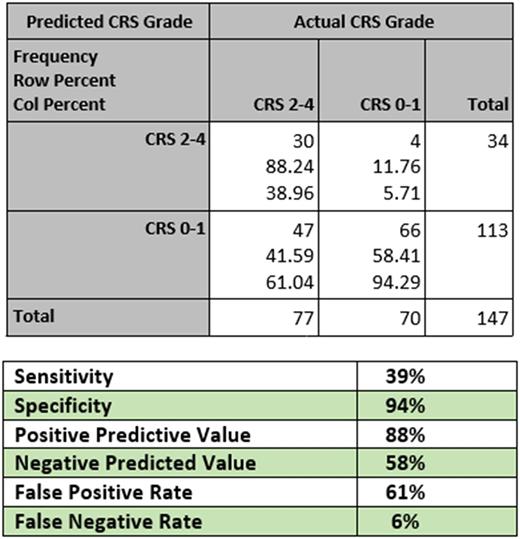
We stratified patients into low risk (CRS grades 0-1) or high risk (CRS grades 2-4). This matches treatment guidelines which dictate that all patients with grades 3-4, and select patients with grade 2, CRS should be treated with IL-6 inhibitors or steroids.
Our predictive model had excellent specificity (94%), positive predictive value (88%), and false negative rate (6%). The sensitivity (39%), negative predictive value (58%), and false positive rate (61%) were poor.
Conclusions : We have created the first model for predicting the risk of CRS in haplo-SCT using pre-transplant patient and donor characteristics. Clinically, our model proves extremely reliable to rule patients in for CRS which may require treatment. If a patient is deemed high risk by our model, they have an 88% chance of subsequently developing grade 2-4 CRS. It is less useful for ruling patients out for CRS. If a patient is deemed low risk, they still have a 42% chance of developing grade 2-4 CRS. This model is immediately clinically applicable to identify patients who must be watched very closely for CRS in the post-transplant period. It is further useful in designing CRS prevention trials, as the model can be used as an enrollment criterion to select patients at high risk for CRS who would benefit from a prophylactic approach.
We are currently working with one outside institution to perform external validation of our model. We are looking for other external partners to further improve the model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal